Catalysts are used to accelarate reactions, reduce their energy cost, and selectively produce desired products. Many nanomaterials are catalytic, and some are commercially used for this purpose. Still, much remains unknown about their properties, and especially about the performance of hybrid organic-inorganic materials. We aim to bring clarity to this field.
In our first study, we wanted to understand and quantify the impact organic coatings have on the catalytic activity of gold nanoparticles (NPs). The literature in this field suffered from a crucial limitation – since nanoparticles (NPs) require coatings (ligands) to be stable in solution, studies were only able to compare one coating to another. We wanted to determine the absolute impact of a coating, compared to uncoated, bare, NPs. To do so, we immobilized gold NPs on glass surfaces, using a high-temperature annealing process to stabilize them while removing organic coatings. We measured the catalytic activity of such immobilized NPs; while we coated others with organic ligands and evaluated their performance. In this way, we were able to quantify the impact a variety of commonly used ligands have on activity, and demonstrated the slow process of ligand removal in some reaction conditions (Langer and Kedem, J. Phys. Chem. C, 2022, 126, 13705–13713).

We then took this study one step further – previously, all of the ligands we tested inhibited the catalytic activity. Can a coating increase it? Turns out, yes! A thin (<1 nm) coating of a cationic polymer, PAH, actually doubles (or more) the catalytic activity for anionic reactants. We demonstrated the effect for a transfer hydrogenation reaction, where the activation energy was lowered by half for the coated catalyst. The enhancement effect is also present for an oxidation reaction, so it seems it will be widely applicable. This study is the first time such an enhancement is conclusively proven, and we are exicted to see how we can further build on these findings (Langer, LeGrand and Kedem, ACS Appl. Mater. Interfaces 2023, 15, 29160–29169).

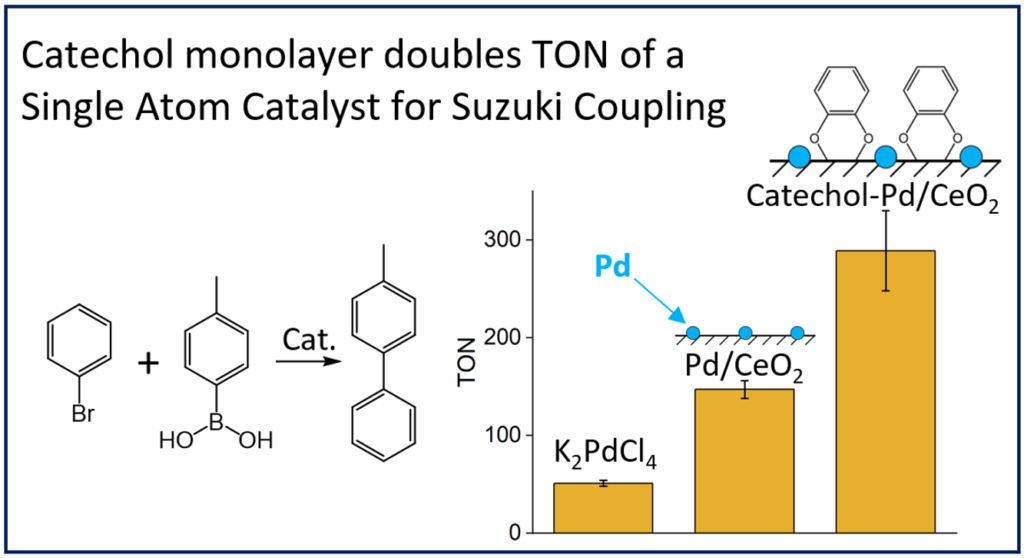
Next, we turned our attention to a different class of catalysts – single atom catalysts (SACs), which are composed of individual catalytic atoms dispersed on supports, such as metal oxides. These atomically dispersed catalysts are highly active, provide high atom economy (all atoms are exposed and reactive, unlike atoms inside the bulk of a nanoparticle), and the micron-sized support particles are easily separated from the reaction mixture by filtration. In many ways, SACs combine the best properties of homogeneous and heterogeneous catalysts, but they lack one crucial property: in homogeneous catalysts like transition-metal catalysts, organic ligands are used to tune the activity and selectivity of the catalysts by interactions with the reactants and catalytic atom, whereas SACs, until now, have used no organic ligands. We decided to explore how ligands attached to the support might affect the catalytic performance of a Pd/CeO2 catalyst toward Suzuki cross-coupling, one of the most widely used reactions in organic synthesis. We found a four-fold acceleration, driven by a halving of the activation energy, likely due to pi-stacking between the ligands (catechols) and the reactants (Audrey Vice, Nicholas Langer, Benjamin Reinhart, and Ofer Kedem. Inorg. Chem. 2023, 62, 21479–21486).